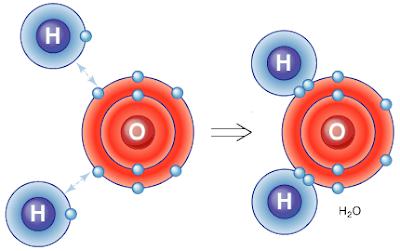
One might wonder, if hydrogen is a highly combustible gas, while oxygen helps it in combustion, then why does the combination of both of these elements as H2O or water have the ability to extinguish a fire?
The answer lies in the difference between mixture and compound found in H2O. At the end of the chemical combination of H20, the form of substance changes completely. The properties become entirely different from the properties of its constituent elements. These constituent elements of the compound cannot be separated easily. The accompanying diagram below gives an idea of how the atom of oxygen binds strongly with the two atoms of hydrogen to form H20. Each atom of oxygen has approximately six electrons that are orbiting around it, but it still has room for two more electrons. If two suitable electrons of some other substance are available near the oxygen, then the element accepts, thus forming a permanent bond. On the other hand, a hydrogen atom has only one electron in its composition. Since the element has only one electron, two atoms of hydrogen fill the gap on the oxygen atom using one electron for each atom. This bounding of atoms of both oxygen and hydrogen elements create the chemical called H2O or water that is abundant on Earth. These bounded atoms of oxygen and hydrogen no longer retain their original properties, hence the reason why they are different from singular oxygen and hydrogen.
Because of the special composition of water, it is able to act as an extinguisher of fire. Water can break the contact between the burning material and the air in the atmosphere, which is what allows the fire to continue blazing. Combustions of fire often stop without the oxygen found in the air. However, there is a situation where water is not quite effective in putting out a fire. If the burning material is a form of liquid, then the weight of the water that will be used to extinguish it should be less than that of the burning liquid. A combustible liquid like petrol is lighter than water, so the fire that is coming from burning petrol cannot be extinguished easily with only using water. If the water is heavier, it only settles below the layer of petrol, and if the petrol remains at the top, it will continue to be in contact with the air that fuels the fire even more.
The same scenario also happens when you mix cooking oil and water in a glass. You would notice that the cooking oil floats entirely at the top while the water stays at the bottom. Even though they are evenly distributed in terms of weight and volume, water is denser than oil, and the denser the liquid is, the more it sinks to the bottom of a container. Of course, water isn’t the densest liquid on Earth, as there are others that are denser, such as liquid soap, corn syrup, and honey. The densest liquid on Earth is mercury.
Water is an exemplary fire extinguisher because there is no substance that can match its ability to absorb heat. In fact, it can even absorb a tremendous amount of heat before it starts evaporating. To show water’s capabilities to absorb heat, let us assume that we have one pound each of gold, iron, and water that are completely devoid of heat, which means that the temperature of each material is about 273 degrees Celsius below zero. Scientifically, the temperature stated is considered to be absolute zero mainly because the substance does not have any heat by that point. Obviously, when water is situated in the said temperature, it will be in the form of ice. While heating these substances, the heat that they get while in a container is evenly absorbed by all of them; however, they will have different melting points. We will find out in the experiment that gold starts melting first at 1102 degrees Celsius, but at this stage, the temperature on the ice is no more than 149 degrees Celsius below zero. When iron starts melting at 1299 degrees Celsius, the inner temperature of the ice barely even reaches 0º Celsius, which is an amazing feat for the object. Ice would start melting gradually after the outside temperature reaches 1300 degrees Celsius, and it will then take the form of water once again. After ice turns to water, the liquid will still take more time and heat before it starts boiling and evaporating.
Before we conclude this article, let us consider some figures again. An ordinary piece of paper catches fire at 184 degrees Celsius, Cotton burns at 228 degrees, and the combustion point of wood or the period where wood would start burning is in the range of 240 degrees to 269 degrees Celsius. All of these burning points can be matched by water, which can easily extinguish these burning objects, whatever their temperature might be.